Accelerating a New Era in Oncology Drug Development and Precision Medicine

SCIENCE
Transforming Cancer Care
The Kibur Medical technology provides a comprehensive molecular profile of the in vivo response of a tumor to up to 20 different oncology drugs or drug combinations simultaneously.
With this information drug developers can design better tolerated, more potent oncology drugs or drug combinations with an increased chance of clinical success and biomarkers predictive of drug efficacy.
Direct measurement of tumor response to different oncology drugs or drug combinations helps identify effective therapies for patients who do not respond to the standard-of-care treatment.
Today’s Challenge
Current translational models in pre-clinical oncology development do not accurately replicate the full complexity of human tumor biology. This results in incomplete knowledge of the tumor-drug response and therefore significantly increases the risk and cost of failure by shifting risk from pre-clinical to clinical development.
Our Solution
We have engineered an innovative, tumor-implanted, drug-eluting NanoNail™ for simultaneous in situ molecular profiling of tumor response to up to 20 different individual oncology drugs and drug combinations.
After retrieval and processing, the resulting multi-omic, spatially registered, single-cell data is analyzed with advanced machine learning and bioinformatic algorithms to provide a deep, comprehensive understanding of the molecular mechanisms of drug-tumor interaction for each drug or drug combination. With the ability to measure in vivo functional activity of multiple compounds simultaneously, the Kibur solution minimizes development risk, reduces clinical attrition, and saves costs.
NanoNail™ is loaded with different drugs or drug combinations
NanoNail™ is implanted
Processing retrieves NanoNail™ and surrounding tumor tissue
Multiomic Tumor Drug Response Measurement
Histological data is collected and analyzed for drug response measurement
50+
Different Proteins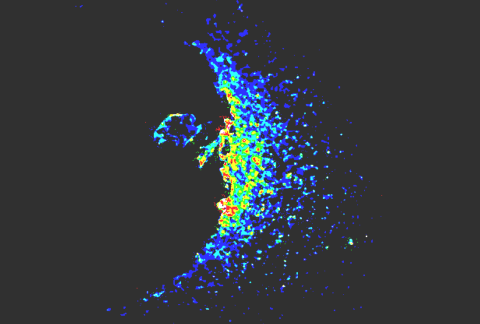
18,000+
Genes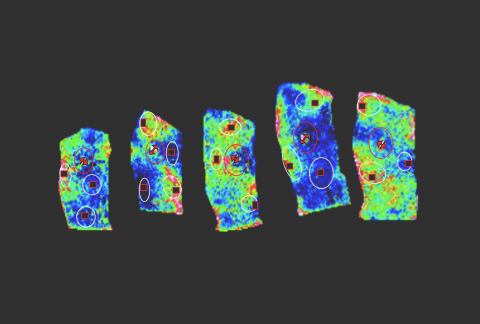
300+
Metabolites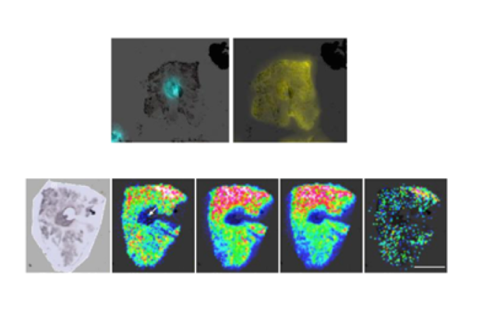
Applications
Discover Optimal Drug Combinations
Determine efficacious drug combinations by screening up to 20 different drug combinations at once in a single tumor simultaneously.
Accelerate Lead Discovery with in vivo High Content Screening
Lead compound discovery from in vivo high content screening of up to 20 compounds at once in a physiologically relevant tumor microenvironment.
Directly Confirm Drug Mechanism of Action.
Spatially registered, single cell, multi-omic profiling provides deep molecular information on tumor response to different drugs or drug combinations.
Identify Predictive Biomarkers
Discover biomarkers as key indicators of drug activity in a physiologically relevant tumor environment.
Human Clinical Testing to Fail Early
Gain early insight into lead molecules efficacy prior to commitment to phased clinical trials.
Key Advantages
Scientifically Proven
Extensive scientific and clinical validation with 20+ publications in high profile, peer-reviewed journals.
Unprecedented Insight
In vivo, multiomic, spatially registered, single-cell data analyzed with advanced bioinformatic tools delivers a comprehensive, multi-dimensional molecular view of the tumor interaction for all tested drugs or drug combinations.
Multiplex Analysis
Simultaneous testing of up to 20 different drug combinations in an intact tumor reveals unique and efficacious combination therapies, especially for immuno-oncology applications.
Clinical De-risking
In situ tumor drug sensitivity profiling in both human tumors and animal tumor models provides early insight into the potential clinical performance of lead molecules.
Patient Centric
Prediction of clinical response from in situ testing of tumor response to different oncology drugs or drug combinations can potentially allow selection of the most efficacious therapy, including standard-of-care, that improves patient outcome.
Clinically Tested
Received IDE approval and is under clinical evaluation in seven FDA-approved clinical studies.
In The Press
Oliver Jonas, our scientific founder, named an awardee of an American Brain Tumor Association Grant to accelerate brain tumor research.
"With more than $35 million invested across 800 research projects, the ABTA is at the forefront of driving progress and seeding hope for life-changing treatments and care for brain tumor patients."
read press releaseFour ways research aims to outwit cancer’s evasion tactics
"They developed an implantable device that can test up to 18 drug combinations simultaneously. . . capturing a more detailed and naturalistic view of the efficacy and mechanism of action for these therapies. This could streamline the vetting process to minimize the risk of testing fruitless pairs."
read press releaseNanoNail reviewed in NIH Director’s Blog
"When implanted into cancerous brain tissue during surgery, the rice-sized drug-releasing device can simultaneously conduct experiments to measure a tumor’s response to more than a dozen drugs or drug combinations."
read press releaseIEEE Pulse Entrepreneurs Corner Episode 1: Colin Brenan Part – 1
"IEEE Pulse: Entrepreneurs Corner podcast episode featuring Dr. Colin Brenan, a serial life sciences entrepreneur and senior executive of biomedical and biotech companies. Dr. Brenan shares insights gained over 35 years of establishing businesses in the biotech and life sciences fields. This episode is Part 1 of 2."
read press releaseKibur Medical announces a strategic partnership with Charles River Laboratories to advance preclinical oncology studies
"Implantable microdevice provides unique insight into individual and combination therapy efficacy during early-stage discovery"
read press releaseLeadership
Kibur Medical is a privately held company based in Boston, Learn more about our current job opportunities and how you can join our talented team of scientists. Together, we are poised to transform cancer care and accelerate a new era in precision oncology drug and diagnostic development.




